A Bone is Able to Continue to Grow in Thickness Throughout Life Due to the Pressence of What
Open access peer-reviewed chapter
Bone Development and Growth
Submitted: September 12th, 2018 Reviewed: November 8th, 2018 Published: December 14th, 2018
DOI: 10.5772/intechopen.82452
IntechOpen Downloads
6,431
Total Chapter Downloads on intechopen.com
Altmetric score
Overall attention for this chapters
Abstract
The process of bone formation is called osteogenesis or ossification. After progenitor cells form osteoblastic lines, they proceed with three stages of development of cell differentiation, called proliferation, maturation of matrix, and mineralization. Based on its embryological origin, there are two types of ossification, called intramembranous ossification that occurs in mesenchymal cells that differentiate into osteoblast in the ossification center directly without prior cartilage formation and endochondral ossification in which bone tissue mineralization is formed through cartilage formation first. In intramembranous ossification, bone development occurs directly. In this process, mesenchymal cells proliferate into areas that have high vascularization in embryonic connective tissue in the formation of cell condensation or primary ossification centers. This cell will synthesize bone matrix in the periphery and the mesenchymal cells continue to differentiate into osteoblasts. After that, the bone will be reshaped and replaced by mature lamellar bone. Endochondral ossification will form the center of primary ossification, and the cartilage extends by proliferation of chondrocytes and deposition of cartilage matrix. After this formation, chondrocytes in the central region of the cartilage start to proceed with maturation into hypertrophic chondrocytes. After the primary ossification center is formed, the marrow cavity begins to expand toward the epiphysis. Then the subsequent stages of endochondral ossification will take place in several zones of the bone.
Keywords
- osteogenesis
- ossification
- bone formation
- intramembranous ossification
- endochondral ossification
*Address all correspondence to: dr_setia76@yahoo.co.id
1. Introduction
Bone is living tissue that is the hardest among other connective tissues in the body, consists of 50% water. The solid part remainder consisting of various minerals, especially 76% of calcium salt and 33% of cellular material. Bone has vascular tissue and cellular activity products, especially during growth which is very dependent on the blood supply as basic source and hormones that greatly regulate this growth process. Bone-forming cells, osteoblasts, osteoclast play an important role in determining bone growth, thickness of the cortical layer and structural arrangement of the lamellae.
Bone continues to change its internal structure to reach the functional needs and these changes occur through the activity of osteoclasts and osteoblasts. The bone seen from its development can be divided into two processes: first is the intramembranous ossification in which bones form directly in the form of primitive mesenchymal connective tissue, such as the mandible, maxilla and skull bones. Second is the endochondral ossification in which bone tissue replaces a preexisting hyaline cartilage, for example during skull base formation. The same formative cells form two types of bone formation and the final structure is not much different.
Bone growth depends on genetic and environmental factors, including hormonal effects, diet and mechanical factors. The growth rate is not always the same in all parts, for example, faster in the proximal end than the distal humerus because the internal pattern of the spongiosum depends on the direction of bone pressure. The direction of bone formation in the epiphysis plane is determined by the direction and distribution of the pressure line. Increased thickness or width of the bone is caused by deposition of new bone in the form of circumferential lamellae under the periosteum. If bone growth continues, the lamella will be embedded behind the new bone surface and be replaced by the haversian canal system.
Advertisement
2. Bone cells and matrix
Bone is a tissue in which the extracellular matrix has been hardened to accommodate a supporting function. The fundamental components of bone, like all connective tissues, are cells and matrix. Although bone cells compose a small amount of the bone volume, they are crucial to the function of bones. Four types of cells are found within bone tissue: osteoblasts, osteocytes, osteogenic cells, and osteoclasts. They each unique functions and are derived from two different cell lines (Figure 1 and Table 1) [1, 2, 3, 4, 5, 6, 7].
-
Osteoblast synthesizes the bone matrix and are responsible for its mineralization. They are derived from osteoprogenitor cells, a mesenchymal stem cell line.
-
Osteocytes are inactive osteoblasts that have become trapped within the bone they have formed.
-
Osteoclasts break down bone matrix through phagocytosis. Predictably, they ruffled border, and the space between the osteoblast and the bone is known as Howship's lacuna.
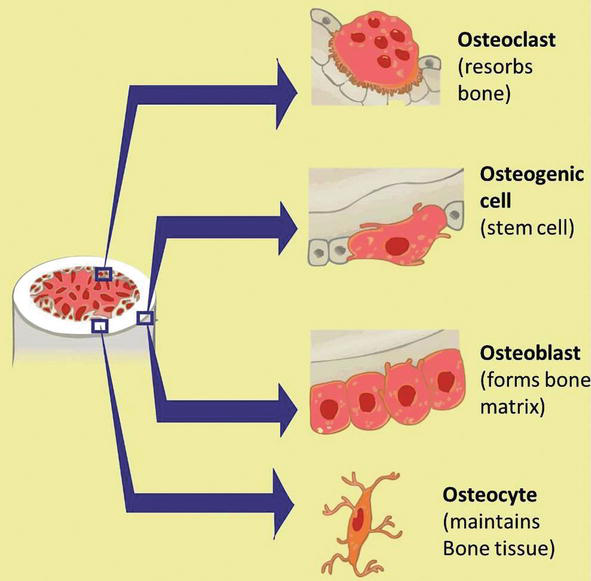
Figure 1.
Development of bone precursor cells. Bone precursor cells are divided into developmental stages, which are 1. mesenchymal stem cell, 2. pre-osteoblast, 3. osteoblast, and 4. mature osteocytes, and 5. osteoclast.
Table 1.
Bone cells, their function, and locations [1, 2, 3, 4, 5, 6, 7].
The balance between osteoblast and osteoclast activity governs bone turnover and ensures that bone is neither overproduced nor overdegraded. These cells build up and break down bone matrix, which is composed of:
-
Osteoid, which is the unmineralized matrix composed of type I collagen and gylcosaminoglycans (GAGs).
-
Calcium hydroxyapatite, a calcium salt crystal that give bone its strength and rigidity.
Bone is divided into two types that are different structurally and functionally. Most bones of the body consist of both types of bone tissue (Figure 2) [1, 2, 8, 9]:
-
Compact bone, or cortical bone, mainly serves a mechanical function. This is the area of bone to which ligaments and tendons attach. It is thick and dense.
-
Trabecular bone, also known as cancellous bone or spongy bone, mainly serves a metabolic function. This type of bone is located between layers of compact bone and is thin porous. Location within the trabeculae is the bone marrow.

Figure 2.
Structure of a long bone.
Advertisement
3. Bone structure
3.1 Macroscopic bone structure
Long bones are composed of both cortical and cancellous bone tissue. They consist of several areas (Figure 3) [3, 4]:
-
The epiphysis is located at the end of the long bone and is the parts of the bone that participate in joint surfaces.
-
The diaphysis is the shaft of the bone and has walls of cortical bone and an underlying network of trabecular bone.
-
The epiphyseal growth plate lies at the interface between the shaft and the epiphysis and is the region in which cartilage proliferates to cause the elongation of the bone.
-
The metaphysis is the area in which the shaft of the bone joins the epiphyseal growth plate.
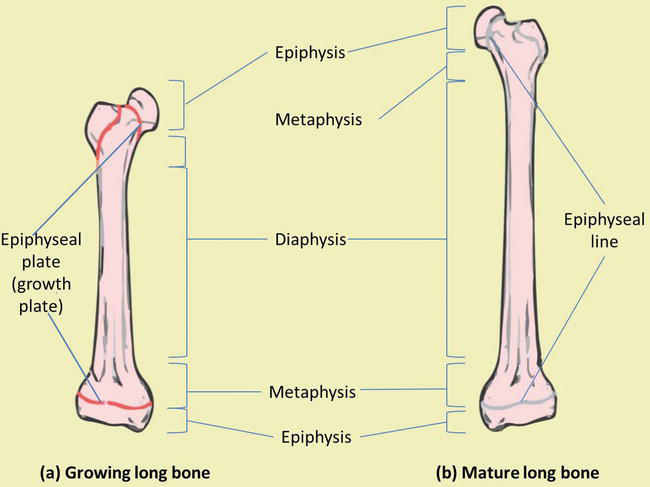
Figure 3.
Bone macrostructure. (a) Growing long bone showing epiphyses, epiphyseal plates, metaphysis and diaphysis. (b) Mature long bone showing epiphyseal lines.
Different areas of the bone are covered by different tissue [4]:
-
The epiphysis is lined by a layer of articular cartilage, a specialized form of hyaline cartilage, which serves as protection against friction in the joints.
-
The outside of the diaphysis is lined by periosteum, a fibrous external layer onto which muscles, ligaments, and tendons attach.
-
The inside of the diaphysis, at the border between the cortical and cancellous bone and lining the trabeculae, is lined by endosteum.
3.2 Microscopic bone structure
Compact bone is organized as parallel columns, known as Haversian systems, which run lengthwise down the axis of long bones. These columns are composed of lamellae, concentric rings of bone, surrounding a central channel, or Haversian canal, that contains the nerves, blood vessels, and lymphatic system of the bone. The parallel Haversian canals are connected to one another by the perpendicular Volkmann's canals.
The lamellae of the Haversian systems are created by osteoblasts. As these cells secrete matrix, they become trapped in spaces called lacunae and become known as osteocytes. Osteocytes communicate with the Haversian canal through cytoplasmic extensions that run through canaliculi, small interconnecting canals (Figure 4) [1, 2, 8, 9]:
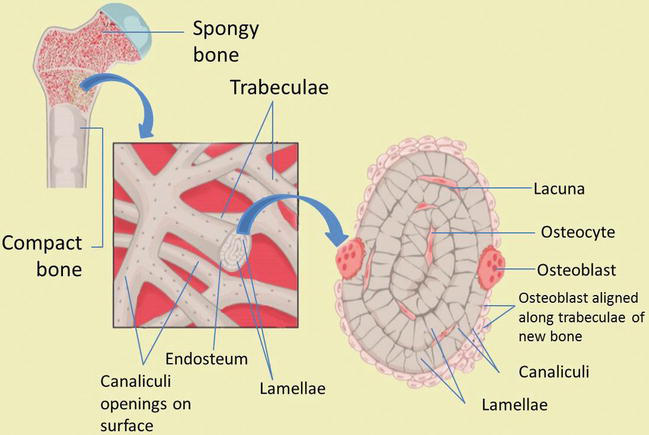
Figure 4.
Bone microstructure. Compact and spongy bone structures.
The layers of a long bone, beginning at the external surface, are therefore:
-
Periosteal surface of compact bone
-
Outer circumferential lamellae
-
Compact bone (Haversian systems)
-
Inner circumferential lamellae
-
Endosteal surface of compact bone
-
Trabecular bone
Advertisement
4. Bone formation
Bone development begins with the replacement of collagenous mesenchymal tissue by bone. This results in the formation of woven bone, a primitive form of bone with randomly organized collagen fibers that is further remodeled into mature lamellar bone, which possesses regular parallel rings of collagen. Lamellar bone is then constantly remodeled by osteoclasts and osteoblasts. Based on the development of bone formation can be divided into two parts, called endochondral and intramembranous bone formation/ossification [1, 2, 3, 8].
4.1 Intramembranous bone formation
During intramembranous bone formation, the connective tissue membrane of undifferentiated mesenchymal cells changes into bone and matrix bone cells [10]. In the craniofacial cartilage bones, intramembranous ossification originates from nerve crest cells. The earliest evidence of intramembranous bone formation of the skull occurs in the mandible during the sixth prenatal week. In the eighth week, reinforcement center appears in the calvarial and facial areas in areas where there is a mild stress strength [11].
Intramembranous bone formation is found in the growth of the skull and is also found in the sphenoid and mandible even though it consists of endochondral elements, where the endochondral and intramembranous growth process occurs in the same bone. The basis for either bone formation or bone resorption is the same, regardless of the type of membrane involved.
Sometimes according to where the formation of bone tissue is classified as "periosteal" or "endosteal". Periosteal bone always originates from intramembranous, but endosteal bone can originate from intramembranous as well as endochondral ossification, depending on the location and the way it is formed [3, 12].
4.1.1 The stage of intramembranous bone formation
The statement below is the stage of intramembrane bone formation (Figure 5) [3, 4, 11, 12]:
-
An ossification center appears in the fibrous connective tissue membrane. Mesenchymal cells in the embryonic skeleton gather together and begin to differentiate into specialized cells. Some of these cells differentiate into capillaries, while others will become osteogenic cells and osteoblasts, then forming an ossification center.
-
Bone matrix (osteoid) is secreted within the fibrous membrane. Osteoblasts produce osteoid tissue, by means of differentiating osteoblasts from the ectomesenchyme condensation center and producing bone fibrous matrix (osteoid). Then osteoid is mineralized within a few days and trapped osteoblast become osteocytes.
-
Woven bone and periosteum form. The encapsulation of cells and blood vessels occur. When osteoid deposition by osteoblasts continues, the encased cells develop into osteocytes. Accumulating osteoid is laid down between embryonic blood vessels, which form a random network (instead of lamellae) of trabecular. Vascularized mesenchyme condenses on external face of the woven bone and becomes the periosteum.
-
Production of osteoid tissue by membrane cells: osteocytes lose their ability to contribute directly to an increase in bone size, but osteoblasts on the periosteum surface produce more osteoid tissue that thickens the tissue layer on the existing bone surface (for example, appositional bone growth). Formation of a woven bone collar that is later replaced by mature lamellar bone. Spongy bone (diploe), consisting of distinct trabeculae, persists internally and its vascular tissue becomes red marrow.
-
Osteoid calcification: The occurrence of bone matrix mineralization makes bones relatively impermeable to nutrients and metabolic waste. Trapped blood vessels function to supply nutrients to osteocytes as well as bone tissue and eliminate waste products.
-
The formation of an essential membrane of bone which includes a membrane outside the bone called the bone endosteum. Bone endosteum is very important for bone survival. Disruption of the membrane or its vascular tissue can cause bone cell death and bone loss. Bones are very sensitive to pressure. The calcified bones are hard and relatively inflexible.
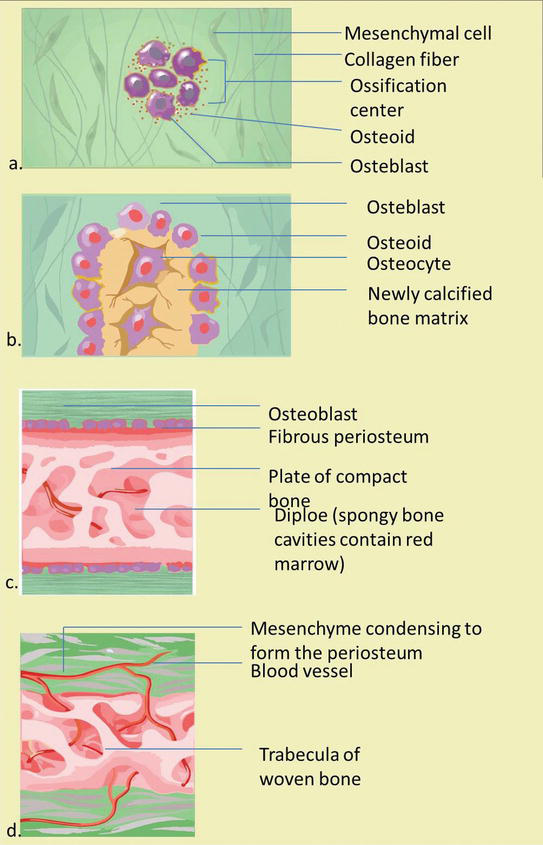
Figure 5.
The stage of intramembranous ossification. The following stages are (a) Mesenchymal cells group into clusters, and ossification centers form. (b) Secreted osteoid traps osteoblasts, which then become osteocytes. (c) Trabecular matrix and periosteum form. (d) Compact bone develops superficial to the trabecular bone, and crowded blood vessels condense into red marrow.
The matrix or intercellular substance of the bone becomes calcified and becomes a bone in the end. Bone tissue that is found in the periosteum, endosteum, suture, and periodontal membrane (ligaments) is an example of intramembranous bone formation [3, 13].
Intramembranous bone formation occurs in two types of bone: bundle bone and lamellar bone. The bone bundle develops directly in connective tissue that has not been calcified. Osteoblasts, which are differentiated from the mesenchyme, secrete an intercellular substance containing collagen fibrils. This osteoid matrix calcifies by precipitating apatite crystals. Primary ossification centers only show minimal bone calcification density. The apatite crystal deposits are mostly irregular and structured like nets that are contained in the medullary and cortical regions. Mineralization occurs very quickly (several tens of thousands of millimeters per day) and can occur simultaneously in large areas. These apatite deposits increase with time. Bone tissue is only considered mature when the crystalized area is arranged in the same direction as collagen fibrils.
Bone tissue is divided into two, called the outer cortical and medullary regions, these two areas are destroyed by the resorption process; which goes along with further bone formation. The surrounding connective tissue will differentiate into the periosteum. The lining in the periosteum is rich in cells, has osteogenic function and contributes to the formation of thick bones as in the endosteum.
In adults, the bundle bone is usually only formed during rapid bone remodeling. This is reinforced by the presence of lamellar bone. Unlike bundle bone formation, lamellar bone development occurs only in mineralized matrix (e.g., cartilage that has calcified or bundle bone spicules). The nets in the bone bundle are filled to strengthen the lamellar bone, until compact bone is formed. Osteoblasts appear in the mineralized matrix, which then form a circle with intercellular matter surrounding the central vessels in several layers (Haversian system). Lamella bone is formed from 0.7 to 1.5 microns per day. The network is formed from complex fiber arrangements, responsible for its mechanical properties. The arrangement of apatites in the concentric layer of fibrils finally meets functional requirements. Lamellar bone depends on ongoing deposition and resorption which can be influenced by environmental factors, one of this which is orthodontic treatment.
4.1.2 Factors that influence intramembranous bone formation
Intramembranous bone formation from desmocranium (suture and periosteum) is mediated by mesenchymal skeletogenetic structures and is achieved through bone deposition and resorption [8]. This development is almost entirely controlled through local epigenetic factors and local environmental factors (i.e. by muscle strength, external local pressure, brain, eyes, tongue, nerves, and indirectly by endochondral ossification). Genetic factors only have a nonspecific morphogenetic effect on intramembranous bone formation and only determine external limits and increase the number of growth periods. Anomaly disorder (especially genetically produced) can affect endochondral bone formation, so local epigenetic factors and local environmental factors, including steps of orthodontic therapy, can directly affect intramembranous bone formation [3, 11].
4.2 Endochondral bone formation
During endochondral ossification, the tissue that will become bone is firstly formed from cartilage, separated from the joint and epiphysis, surrounded by perichondrium which then forms the periosteum [11]. Based on the location of mineralization, it can be divided into: Perichondral Ossification and Endochondral Ossification. Both types of ossification play an essential role in the formation of long bones where only endochondral ossification takes place in short bones. Perichondral ossification begins in the perichondrium. Mesenchymal cells from the tissue differentiate into osteoblasts, which surround bony diaphyseal before endochondral ossification, indirectly affect its direction [3, 8, 12]. Cartilage is transformed into bone is craniofacial bone that forms at the eigth prenatal week. Only bone on the cranial base and part of the skull bone derived from endochondral bone formation. Regarding to differentiate endochondral bone formation from chondrogenesis and intramembranous bone formation, five sequences of bone formation steps were determined [3].
4.2.1 The stages of endochondral bone formation
The statements below are the stages of endochondral bone formation (Figure 6) [4, 12]:
-
Mesenchymal cells group to form a shape template of the future bone.
-
Mesenchymal cells differentiate into chondrocytes (cartilage cells).
-
Hypertrophy of chondrocytes and calcified matrix with calcified central cartilage primordium matrix formed. Chondrocytes show hypertrophic changes and calcification from the cartilage matrix continues.
-
Entry of blood vessels and connective tissue cells. The nutrient artery supplies the perichondrium, breaks through the nutrient foramen at the mid-region and stimulates the osteoprogenitor cells in the perichondrium to produce osteoblasts, which changes the perichondrium to the periosteum and starts the formation of ossification centers.
-
The periosteum continues its development and the division of cells (chondrocytes) continues as well, thereby increasing matrix production (this helps produce more length of bone).
-
The perichondrial membrane surrounds the surface and develops new chondroblasts.
-
Chondroblasts produce growth in width (appositional growth).
-
Cells at the center of the cartilage lyse (break apart) triggers calcification.
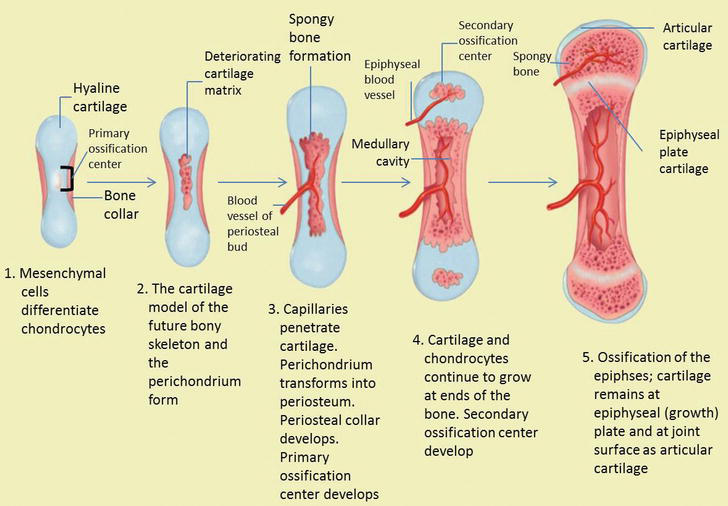
Figure 6.
The stage of endochondral ossification. The following stages are: (a) Mesenchymal cells differentiate into chondrocytes. (b) The cartilage model of the future bony skeleton and the perichondrium form. (c) Capillaries penetrate cartilage. Perichondrium transforms into periosteum. Periosteal collar develops. Primary ossification center develops. (d) Cartilage and chondrocytes continue to grow at ends of the bone. (e) Secondary ossification centers develop. (f) Cartilage remains at epiphyseal (growth) plate and at joint surface as articular cartilage.
During endochondral bone formation, mesenchymal tissue firstly differentiates into cartilage tissue. Endochondral bone formation is morphogenetic adaptation (normal organ development) which produces continuous bone in certain areas that are prominently stressed. Therefore, this endochondral bone formation can be found in the bones associated with joint movements and some parts of the skull base. In hypertrophic cartilage cells, the matrix calcifies and the cells undergo degeneration. In cranial synchondrosis, there is proliferation in the formation of bones on both sides of the bone plate, this is distinguished by the formation of long bone epiphyses which only occurs on one side only [2, 14].
As the cartilage grows, capillaries penetrate it. This penetration initiates the transformation of the perichondrium into the bone-producing periosteum. Here, the osteoblasts form a periosteal collar of compact bone around the cartilage of the diaphysis. By the second or third month of fetal life, bone cell development and ossification ramps up and creates the
While these deep changes occur, chondrocytes and cartilage continue to grow at the ends of the bone (the future epiphyses), which increase the bone length and at the same time bone also replaces cartilage in the diaphysis. By the time the fetal skeleton is fully formed, cartilage only remains at the joint surface as articular cartilage and between the diaphysis and epiphysis as the epiphyseal plate, the latter of which is responsible for the longitudinal growth of bones. After birth, this same sequence of events (matrix mineralization, death of chondrocytes, invasion of blood vessels from the periosteum, and seeding with osteogenic cells that become osteoblasts) occur in the epiphyseal regions, and each of these centers of activity is referred to as a
There are four important things about cartilage in endochondral bone formation:
-
Cartilage has a rigid and firm structure, but not usually calcified nature, giving three basic functions of growth (a) its flexibility can support an appropriate network structure (nose), (b) pressure tolerance in a particular place where compression occurs, (c) the location of growth in conjunction with enlarging bone (synchondrosis of the skull base and condyle cartilage).
-
Cartilage grows in two adjacent places (by the activity of the chondrogenic membrane) and grows in the tissues (chondrocyte cell division and the addition of its intercellular matrix).
-
Bone tissue is not the same as cartilage in terms of its tension adaptation and cannot grow directly in areas of high compression because its growth depends on the vascularization of bone formation covering the membrane.
-
Cartilage growth arises where linear growth is required toward the pressure direction, which allows the bone to lengthen to the area of strength and has not yet grown elsewhere by membrane ossification in conjunction with all periosteal and endosteal surfaces.
4.2.2 Factors that influence endochondral ossification
Membrane disorders or vascular supply problem of these essential membranes can directly result in bone cell death and ultimately bone damage. Calcified bones are generally hard and relatively inflexible and sensitive to pressure [12].
Cranial synchondrosis (e.g., spheno ethmoidal and spheno occipital growth) and endochondral ossification are further determined by chondrogenesis. Chondrogenesis is mainly influenced by genetic factors, similar to facial mesenchymal growth during initial embryogenesis to the differentiation phase of cartilage and cranial bone tissue.
This process is only slightly affected by local epigenetic and environmental factors. This can explain the fact that the cranial base is more resistant to deformation than desmocranium. Local epigenetic and environmental factors cannot trigger or inhibit the amount of cartilage formation. Both of these have little effect on the shape and direction of endochondral ossification. This has been analyzed especially during mandibular condyle growth.
Local epigenetics and environmental factors only affect the shape and direction of cartilage formation during endochondral ossification Considering the fact that condyle cartilage is a secondary cartilage, it is assumed that local factors provide a greater influence on the growth of mandibular condyle.
4.2.3 Chondrogenesis
Chondrogenesis is the process by which cartilage is formed from condensed mesenchyme tissue, which differentiates into chondrocytes and begins secreting the molecules that form the extracellular matrix [5, 14].
The statement below is five steps of chondrogenesis [8, 14]:
-
Chondroblasts produce a matrix: the extracellular matrix produced by cartilage cells, which is firm but flexible and capable of providing a rigid support.
-
Cells become embed in a matrix: when the chondroblast changes to be completely embed in its own matrix material, cartilage cells turn into chondrocytes. The new chondroblasts are distinguished from the membrane surface (perichondrium), this will result in the addition of cartilage size (cartilage can increase in size through apposition growth).
-
Chondrocytes enlarge, divide and produce a matrix. Cell growth continues and produces a matrix, which causes an increase in the size of cartilage mass from within. Growth that causes size increase from the inside is called interstitial growth.
-
The matrix remains uncalcified: cartilage matrix is rich of chondroitin sulfate which is associated with non-collagen proteins. Nutrition and metabolic waste are discharged directly through the soft matrix to and from the cell. Therefore, blood vessels aren't needed in cartilage.
-
The membrane covers the surface but is not essential: cartilage has a closed membrane vascularization called perichondrium, but cartilage can exist without any of these. This property makes cartilage able to grow and adapt where it needs pressure (in the joints), so that cartilage can receive pressure.
Endochondral ossification begins with characteristic changes in cartilage bone cells (hypertrophic cartilage) and the environment of the intercellular matrix (calcium laying), the formation which is called as primary spongiosa. Blood vessels and mesenchymal tissues then penetrate into this area from the perichondrium. The binding tissue cells then differentiate into osteoblasts and cells. Chondroblasts erode cartilage in a cave-like pattern (cavity). The remnants of mineralized cartilage the central part of laying the lamellar bone layer.
The osteoid layer is deposited on the calcified spicules remaining from the cartilage and then mineralized to form spongiosa bone, with fine reticular structures that resemble nets that possess cartilage fragments between the spicular bones. Spongy bones can turn into compact bones by filling empty cavities. Both endochondral and perichondral bone growth both take place toward epiphyses and joints. In the bone lengthening process during endochondral ossification depends on the growth of epiphyseal cartilage. When the epiphyseal line has been closed, the bone will not increase in length. Unlike bone, cartilage bone growth is based on apposition and interstitial growth. In areas where cartilage bone is covered by bone, various variations of zone characteristics, based on the developmental stages of each individual, can differentiate which then continuously merge with each other during the conversion process. Environmental influences (co: mechanism of orthopedic functional tools) have a strong effect on condylar cartilage because the bone is located more superficially [5].
Advertisement
5. Bone growth
Cartilage bone height development occurs during the third month of intra uterine life. Cartilage plate extends from the nasal bone capsule posteriorly to the foramen magnum at the base of the skull. It should be noted that cartilages which close to avascular tissue have internal cells obtained from the diffusion process from the outermost layer. This means that the cartilage must be flatter. In the early stages of development, the size of a very small embryo can form a chondroskeleton easily in which the further growth preparation occurs without internal blood supply [1].
During the fourth month in the uterus, the development of vascular elements to various points of the chondrocranium (and other parts of the early cartilage skeleton) becomes an ossification center, where the cartilage changes into an ossification center, and bone forms around the cartilage. Cartilage continues to grow rapidly but it is replaced by bone, resulting in the rapid increase of bone amount. Finally, the old chondrocranium amount will decrease in the area of cartilage and large portions of bone, assumed to be typical in ethmoid, sphenoid, and basioccipital bones. The cartilage growth in relation to skeletal bone is similar as the growth of the limbs [1, 3].
Longitudinal bone growth is accompanied by remodeling which includes appositional growth to thicken the bone. This process consists of bone formation and reabsorption. Bone growth stops around the age of 21 for males and the age of 18 for females when the epiphyses and diaphysis have fused (epiphyseal plate closure).
Normal bone growth is dependent on proper dietary intake of protein, minerals and vitamins. A deficiency of vitamin D prevents calcium absorption from the GI tract resulting in rickets (children) or osteomalacia (adults). Osteoid is produced but calcium salts are not deposited, so bones soften and weaken.
5.1 Oppositional bone growth
At the length of the long bones, the reinforcement plane appears in the middle and at the end of the bone, finally produces the central axis that is called the diaphysis and the bony cap at the end of the bone is called the epiphysis. Between epiphyses and diaphysis is a calcified area that is not calcified called the epiphyseal plate. Epiphyseal plate of the long bone cartilage is a major center for growth, and in fact, this cartilage is responsible for almost all the long growths of the bones. This is a layer of hyaline cartilage where ossification occurs in immature bones. On the epiphyseal side of the epiphyseal plate, the cartilage is formed. On the diaphyseal side, cartilage is ossified, and the diaphysis then grows in length. The epiphyseal plate is composed of five zones of cells and activity [3, 4].
Near the outer end of each epiphyseal plate is the active zone dividing the cartilage cells. Some of them, pushed toward diaphysis with proliferative activity, develop hypertrophy, secrete an extracellular matrix, and finally the matrix begins to fill with minerals and then is quickly replaced by bone. As long as cartilage cells multiply growth will continue. Finally, toward the end of the normal growth period, the rate of maturation exceeds the proliferation level, the latter of the cartilage is replaced by bone, and the epiphyseal plate disappears. At that time, bone growth is complete, except for surface changes in thickness, which can be produced by the periosteum [4]. Bones continue to grow in length until early adulthood. The lengthening is stopped in the end of adolescence which chondrocytes stop mitosis and plate thins out and replaced by bone, then diaphysis and epiphyses fuse to be one bone (Figure 7). The rate of growth is controlled by hormones. When the chondrocytes in the epiphyseal plate cease their proliferation and bone replaces the cartilage, longitudinal growth stops. All that remains of the epiphyseal plate is the epiphyseal line. Epiphyseal plate closure will occur in 18-year old females or 21-year old males.

Figure 7.
Oppositional bone growth and remodeling. The epiphyseal plate is responsible for longitudinal bone growth.
5.1.1 Epiphyseal plate growth
The cartilage found in the epiphyseal gap has a defined hierarchical structure, directly beneath the secondary ossification center of the epiphysis. By close examination of the epiphyseal plate, it appears to be divided into five zones (starting from the epiphysis side) (Figure 8) [4]:
-
The resting zone: it contains hyaline cartilage with few chondrocytes, which means no morphological changes in the cells.
-
The proliferative zone: chondrocytes with a higher number of cells divide rapidly and form columns of stacked cells parallel to the long axis of the bone.
-
The hypertrophic cartilage zone: it contains large chondrocytes with cells increasing in volume and modifying the matrix, effectively elongating bone whose cytoplasm has accumulated glycogen. The resorbed matrix is reduced to thin septa between the chondrocytes.
-
The calcified cartilage zone: chondrocytes undergo apoptosis, the thin septa of cartilage matrix become calcified.
-
The ossification zone: endochondral bone tissue appears. Blood capillaries and osteoprogenitor cells (from the periosteum) invade the cavities left by the chondrocytes. The osteoprogenitor cells form osteoblasts, which deposit bone matrix over the three-dimensional calcified cartilage matrix.
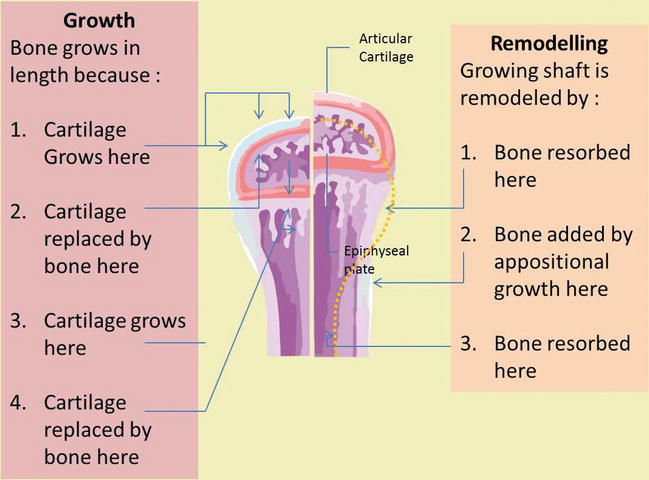
Figure 8.
Epiphyseal plate growth. Five zones of epiphyseal growth plate includes: 1. resting zone, 2. proliferation zone, 3. hypertrophic cartilage zone, 4. calcified cartilage zone, and 5. ossification zone.
5.2 Appositional bone growth
When bones are increasing in length, they are also increasing in diameter; diameter growth can continue even after longitudinal growth stops. This is called appositional growth. The bone is absorbed on the endosteal surface and added to the periosteal surface. Osteoblasts and osteoclasts play an essential role in appositional bone growth where osteoblasts secrete a bone matrix to the external bone surface from diaphysis, while osteoclasts on the diaphysis endosteal surface remove bone from the internal surface of diaphysis. The more bone around the medullary cavity is destroyed, the more yellow marrow moves into empty space and fills space. Osteoclasts resorb the old bone lining the medullary cavity, while osteoblasts through intramembrane ossification produce new bone tissue beneath the periosteum. Periosteum on the bone surface also plays an important role in increasing thickness and in reshaping the external contour. The erosion of old bone along the medullary cavity and new bone deposition under the periosteum not only increases the diameter of the diaphysis but also increases the diameter of the medullary cavity. This process is called modeling (Figure 9) [3, 4, 15].

Figure 9.
Appositional bone growth. Bone deposit by osteoblast as bone resorption by osteoclast.
Advertisement
6. The role of mesenchymal stem cell migration and differentiation in bone formation
Recent research reported that bone microstructure is also the principle of bone function, which regulates its mechanical function. Bone tissue function influenced by many factors, such as hormones, growth factors, and mechanical loading. The microstructure of bone tissue is distribution and alignment of biological apatite (BAp) crystallites. This is determined by the direction of bone cell behavior, for example cell migration and cell regulation. Ozasa et al. found that artificial control the direction of mesenchymal stem cell (MSCs) migration and osteoblast alignment can reconstruct bone microstructure, which guide an appropriate bone formation during bone remodeling and regeneration [16].
Bone development begins with the replacement of collagenous mesenchymal tissue by bone. Generally, bone is formed by endochondral or intramembranous ossification. Intramembranous ossification is essential in the bone such as skull, facial bones, and pelvis which MSCs directly differentiate to osteoblasts. While, endochondral ossification plays an important role in most bones in the human skeleton, including long, short, and irregular bones, which MSCs firstly experience to condensate and then differentiate into chondrocytes to form the cartilage growth plate and the growth plate is then gradually replaced by new bone tissue [3, 8, 12].
MSC migration and differentiation are two important physiological processes in bone formation. MSCs migration raise as an essential step of bone formation because MSCs initially need to migrate to the bone surface and then contribute in bone formation process, although MSCs differentiation into osteogenic cells is also crucial. MSC migration during bone formation has attracted more attention. Some studies show that MSC migration to the bone surface is crucial for bone formation [17]. Bone marrow and periosteum are the main sources of MSCs that participate in bone formation [18].
In the intramembranous ossification, MSCs undergo proliferation and differentiation along the osteoblastic lineage to form bone directly without first forming cartilage. MSC and preosteoblast migration is involved in this process and are mediated by plentiful factors in vivo and in vitro. MSCs initially differentiate into preosteoblasts which proliferate near the bone surface and secrete ALP. Then they become mature osteoblasts and then form osteocytes which embedded in an extracellular matrix (ECM). Other factors also regulate the intramembranous ossification of MSCs such as Runx2, special AT-rich sequence binding protein 2 (SATB 2), and Osterix as well as pathways, like the wnt/β-catenin pathway and bone morphogenetic protein (BMP) pathway [17, 19].
In the endochondral ossification, MSCs are first condensed to initiate cartilage model formation. The process is mediated by BMPs through phosphorylating and activating receptor SMADs to transduce signals. During condensation, the central part of MSCs differentiates into chondrocytes and secretes cartilage matrix. While, other cells in the periphery, form the perichondrium that continues expressing type I collagen and other important factors, such as proteoglycans and ALP. Chondrocytes undergo rapid proliferation. Chondrocytes in the center become maturation, accompanied with an invasion of hypertrophic cartilage by the vasculature, followed by differentiation of osteoblasts within the perichondrium and marrow cavity. The inner perichondrium cells differentiate into osteoblasts, which secrete bone matrix to form the bone collar after vascularization in the hypertrophic cartilage. Many factors that regulate endochondral ossification are growth factors (GFs), transforming growth factor-β (TGF-β), Sry-related high-mobility group box 9 (Sox9) and Cell-to-cell interaction [17, 19].
Advertisement
7. Conclusions
-
Osteogenesis/ossification is the process in which new layers of bone tissue are placed by osteoblasts.
-
During bone formation, woven bone (haphazard arrangement of collagen fibers) is remodeled into lamellar bones (parallel bundles of collagen in a layer known as lamellae)
-
Periosteum is a connective tissue layer on the outer surface of the bone; the endosteum is a thin layer (generally only one layer of cell) that coats all the internal surfaces of the bone
-
Major cell of bone include: osteoblasts (from osteoprogenitor cells, forming osteoid that allow matrix mineralization to occur), osteocytes (from osteoblasts; closed to lacunae and retaining the matrix) and osteoclasts (from hemopoietic lineages; locally erodes matrix during bone formation and remodeling.
-
The process of bone formation occurs through two basic mechanisms:
-
Intramembranous bone formation occurs when bone forms inside the mesenchymal membrane. Bone tissue is directly laid on primitive connective tissue referred to mesenchyma without intermediate cartilage involvement. It forms bone of the skull and jaw; especially only occurs during development as well as the fracture repair.
-
Endochondral bone formation occurs when hyaline cartilage is used as a precursor to bone formation, then bone replaces hyaline cartilage, forms and grows all other bones, occurs during development and throughout life.
-
-
During interstitial epiphyseal growth (elongation of the bone), the growth plate with zonal organization of endochondral ossification, allows bone to lengthen without epiphyseal growth plates enlarging zones include:
-
Zone of resting.
-
Zone of proliferation.
-
Zone of hypertrophy.
-
Zone of calcification.
-
Zone of ossification and resorption.
-
-
During appositional growth, osteoclasts resorb old bone that lines the medullary cavity, while osteoblasts, via intramembranous ossification, produce new bone tissue beneath the periosteum.
-
Mesenchymal stem cell migration and differentiation are two important physiological processes in bone formation.
Advertisement
Acknowledgments
The author is grateful to Zahrona Kusuma Dewi for assistance with preparation of the manuscript.
Advertisement
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Advertisement
Acronyms and abbreviations
alkaline phosphatase
biological apatite
bone morphogenetic protein
extracellular matrix
growth factors
mesenchymal stem cells
runt-related transcription factor 2
special AT-rich sequence binding protein 2
sry-related high-mobility group box 9
transforming growth factor-β
References
- 1.
Vanputte CL, Regan JL, Russo AF. Skeletal system: Bones and joints. In: Seeley's Essentials of Anatomy & Physiology. 8th ed. USA: Mc Graw Hill; 2013. pp. 110-149 - 2.
Muscolino JE. Kinesiology the Skelatal System and Muscle Function. 2nd ed. New York: Elsevier Inc.; 2011 - 3.
Cashman KD, Ginty F. Bone. New York: Elsevier; 2003. pp. 1106-1112 - 4.
OpenStax College. Anatomy & Physiology. Texas: Rice University; 2013. pp. 203-231 - 5.
Florencia-Silva R, Rodrigues G, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biolmed Research International. 2015:1-17 - 6.
Tim A. Bone Structure and Bone Remodelling. London: University College London; 2014 - 7.
Mohamed AM. Review article an overview of bone cells and their regulating. Malaysian Journal of Medical Sciences. 2008; 15 (1):4-12 - 8.
Akter F, Ibanez J. Bone and cartilage tissue engineering. In: Akter F, editor. Tissue Engineering Made Easy [Internet]. 1st ed. New York: Elsevier Inc.; 2016. pp. 77-98. DOI: 10.1016/B978-0-12-805361-4.00008-4 - 9.
Guus van der Bie MD, editor. Morphological Anatomy from a Phenomenological Point of View. Rome: Louis Bolk Institute; 2012 - 10.
Wojnar R. Bone and Cartilage–Its Structure and Physical Properties.Weinheim: Wiley-VCH Verlag GmbH & Co.; 2010 - 11.
Karaplis AC. Embryonic development of bone and regulation of intramembranous and endochondral bone formation. In: Bilezikian J, Raisz L, Martin TJ, editors. Principles of Bone Biology. 3rd ed. New York: Academic Press; 2008. pp. 53-84 - 12.
Dennis SC, Berkland CJ, Bonewald LF, Determore MS. Endochondral ossification for enhancing bone regeneration: Converging native ECM biomaterials and developmental engineering in vivo. Tissue Engineering. Part B, Reviews. 2015; 21 (3):247-266 - 13.
Clarke B. Normal bone anatomy and physiology. Clinical Journal of the American Society of Nephrology. 2008; 3 :131-139 - 14.
Provot S, Schipani E, Wu JY, Kronenberg H. Development of the skeleton. In: Marcus R, Dempster D, Cauley J, Feldman D, editors. Osteoporosis [Internet]. 4th ed. New York: Elsevier; 2013. pp. 97-126. DOI: 10.1016/B978-0-12-415853-5.00006-6 - 15.
Schindeler A, Mcdonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Seminars in Cell & Developmental Biology. 2008; 19 :459-466. DOI. 10.1016/j.semcdb.2008.07.004 - 16.
Ozasa R, Matsugaki A, Isobe Y, Saku T, Yun H-S, Nakano T. Construction of human induced pluripotent stem cell-derived oriented bone matrix microstructure by using in vitro engineered anisotropic culture model. Journal of Biomedial Materials Research Part A. 2018; 106 :360-369 - 17.
Su P, Tian Y, Yang C, Ma X, Wang X. Mesenchymal stem cell migration during bone formation and bone diseases therapy. International Journal of Molecular Sciences. 2018; 19 :2343 - 18.
Wang X, Wang Y, Gou W, Lu Q. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. International Orthopaedics. 2013; 37 :2491-2498 - 19.
Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World Journal of Stem Cells. 2013; 5 (4):136-148
Submitted: September 12th, 2018 Reviewed: November 8th, 2018 Published: December 14th, 2018
© 2018 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
beaversluffird1958.blogspot.com
Source: https://www.intechopen.com/chapters/64747
0 Response to "A Bone is Able to Continue to Grow in Thickness Throughout Life Due to the Pressence of What"
Post a Comment